With the spring season rapidly approaching, I want to take the time to remind everyone the value of stabilizing your nitrogen fertilizer. Now, I predict the weather as well as the next guy. I heard several experts last February predict that 2016 would be just like the drought of 2012. Well, those people have to be feeling pretty foolish this winter due to our irrigated like conditions this past season. The point I’m trying to make is that like the weather, we can’t predict the potential nitrogen losses that we are going to experience this year. So in my mind, it would be wise to try to protect a crop input as valuable as nitrogen.
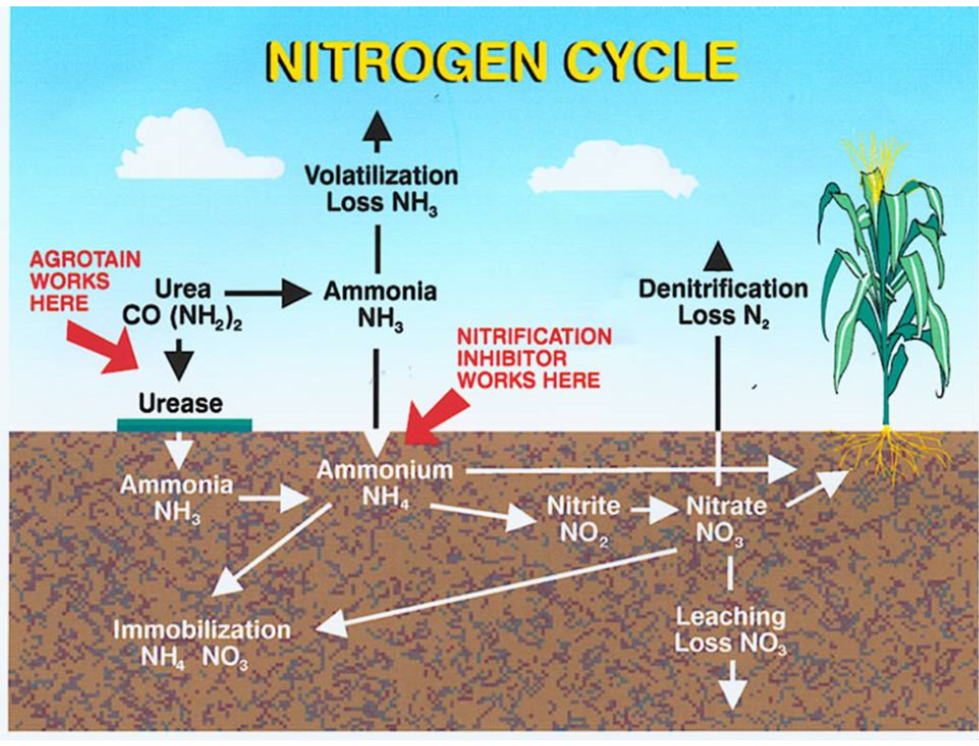
Thinking about the nitrogen cycle, our goal of using stabilizers is to keep our nitrogen inputs in a stable form for as long as possible. As a point of reference, the nitrogen cycle generally follows this pattern: urea is converted to ammonium (NH4+) which then will be converted to nitrate (NO3-). The last two forms can both be taken up by the plant with the difference being ammonium is held by the soil and nitrate is prone to being lost from the plant root zone. I’ll go through some of the major nitrogen fertilizers to discuss how we can protect them. When we inject anhydrous ammonia, it reacts with soil moisture to become ammonium which is stable in the soil. When soil temperatures are above 50°F, soil bacteria will convert ammonium into nitrate over time. With abundant rainfall, nitrate will leach out of well-drained soil. If the soil is poorly drained, the nitrate will denitrify into the atmosphere for as long as the soil is saturated. As many of you know, adding a product like N-serve inhibits the nitrification process, working to keep nitrogen in the ammonium form. Nitrapyrin, the active ingredient in N-serve, targets the Nitrosomonas soil bacteria that convert ammonium and renders them inactive for a period of time. The ammonium will bind with the soil colloid, protecting it from leaching and denitrification when we experience large rain events like the 4 to 7 inch event we received last June. Urea containing fertilizers add one more step to the process where nitrogen losses can occur. Urea combines with water to convert to ammonia (NH3) with the help of the urease enzyme that exists in the soil and on crop residue. If the ammonia isn’t worked into the ground by tillage or rain, it can volatilize into the atmosphere as a gas. As long as the urea is worked into the ground in 2 to 3 days after application, significant volatilization shouldn’t occur. Products like Agrotain or Factor X2 contain NBPT which prevents the urease enzyme from binding to the urea. This gives us 2-3 weeks to work in the urea or wait for adequate rainfall (0.25 in.). Volatilization is mostly likely to occur in these situations: high crop residue, high temperatures, high pH, low organic matter, and wet ground that dries out. UAN solutions have half the volatilization potential as urea because UAN is only half urea with the other half split between ammonium and nitrate. Urea containing fertilizers are subject to leaching and denitrification once they are converted to the nitrate form in the soil and can be protected with a nitrapyrin containing product like Instinct II.
To wrap things up, I can’t say that nitrogen stabilizers will deliver outstanding return on investments every year. As I pointed out earlier though, rainfall and time we have for tillage will vary every year so it would seem to be a wise decision to invest in nitrogen stabilizers as an insurance plan for whatever Mother Nature will throw at us this year. Contact an agronomist at WS Ag Center to discuss how nitrogen stabilizers can be integrated into your farming operation.
– Mark Kendall